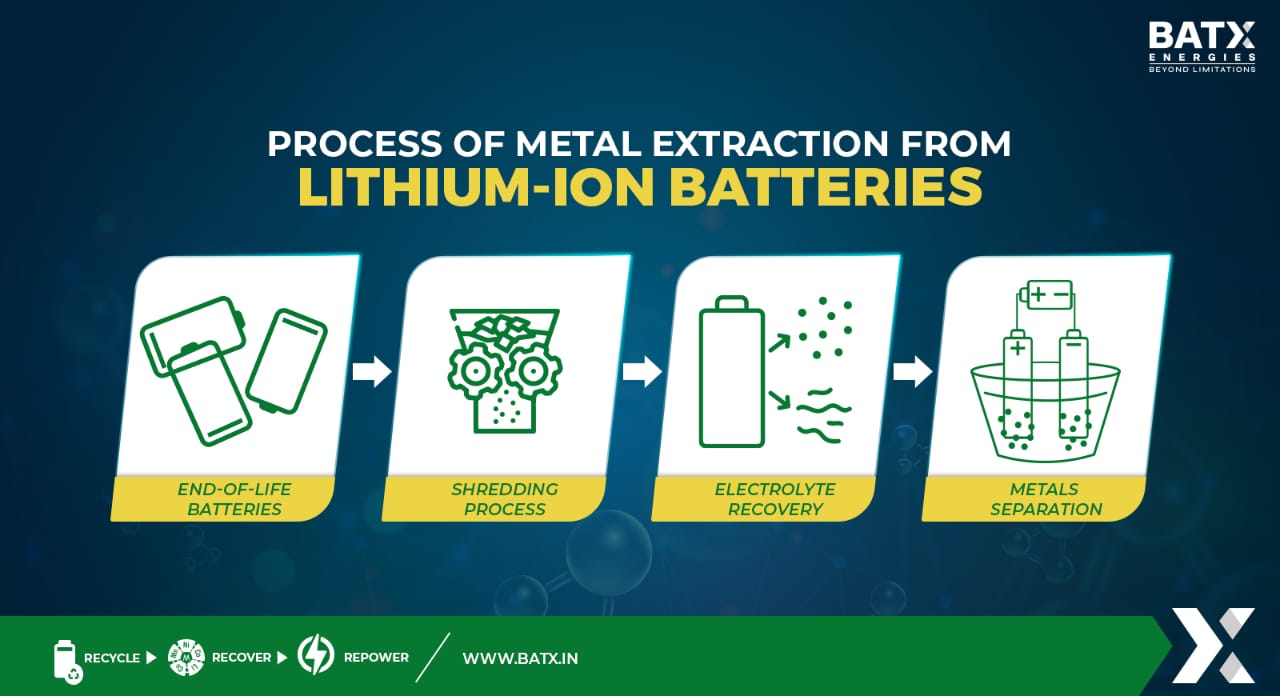
Process of metal extraction from lithium-ion batteries
Lithium-ion batteries have become the preferred choice for portable electronics, electric vehicles, and energy storage systems due to their high energy density and long cycle life. However, as these batteries reach their end of life, it is crucial to properly recycle and extract valuable metals from them. This article will examine the procedure of metal extraction from lithium-ion batteries, emphasizing the significance of recycling and its positive impact on the environment.
|
Table of contents Understanding Lithium-Ion Batteries Collection and Sorting Battery Disassembly Crushing and Shredding Sieving and Separation Leaching Purification and Recovery Metal Refining and Recycling Environmental Benefits of Metal Extraction from Lithium-Ion Batteries |
Understanding Lithium-Ion Batteries
Lithium-ion batteries are composed of several components, including a cathode, an anode, a separator, and an electrolyte. The cathode typically consists of lithium transition metal oxides, such as lithium cobalt oxide (LiCoO2), lithium nickel cobalt aluminum oxide (NCA), or lithium iron phosphate (LiFePO4). The anode is commonly made of graphite, and the electrolyte is a lithium salt dissolved in an organic solvent.
The Metal Extraction Process
Various steps are needed for metal extraction from lithium-ion batteries. Let’s look into them in detail.
Collection and Sorting
The first step in the metal extraction process is the collection and sorting of lithium-ion batteries. These batteries can be collected from various sources, such as recycling centers, electronic waste facilities, and manufacturers. During the sorting stage, batteries are categorized based on their chemistry, size, and type to ensure proper treatment in subsequent processes.
Battery Disassembly
Once sorted, the batteries undergo disassembly. This process involves removing the outer casing and separating the different battery components, including the cathode, anode, and electrolyte. Disassembly can be done manually or through automated processes, depending on the scale of recycling operations.
Crushing and Shredding
After disassembly, the battery components, particularly the cathode and anode, are crushed or shredded into small pieces. This step increases the surface area of the materials, facilitating subsequent chemical processes.
Sieving and Separation
The shredded battery components are sieved to separate different particle sizes. This separation allows for the isolation of valuable materials and helps optimize the efficiency of subsequent extraction processes. Magnetic separation techniques may also be employed to separate ferrous materials, such as steel, from the rest of the components.
Leaching
Leaching is a critical step in the metal extraction process. It involves treating the shredded battery components with chemical solutions to selectively dissolve and extract the desired metals. The choice of leaching agents depends on the specific metals targeted for recovery. For example, acid leaching is commonly used to extract metals like cobalt and nickel, while lithium extraction may require specific solvents or electrolysis.
Purification and Recovery
After leaching, the metal-containing solutions undergo purification to remove impurities and separate different metals. Techniques such as precipitation, solvent extraction, and ion exchange are employed to achieve high-purity metal solutions. These purified solutions can then be further processed to obtain metal salts or subjected to electrolysis for direct metal recovery.
Metal Refining and Recycling
The purified metals are refined to meet the quality requirements for reuse in battery manufacturing or other applications. Refining processes may involve electro-refining, where impurities are selectively removed through electrolysis, or thermal methods like smelting and refining to obtain high-purity metals. The recovered metals can be used to produce new batteries or recycled for various industrial applications, reducing the demand for virgin metals.
Environmental Benefits of Metal Extraction from Lithium-Ion Batteries
The metal extraction from lithium-ion batteries offers significant environmental benefits, including:
Resource Conservation: Metal extraction from spent batteries reduces the need for primary metal mining, conserving valuable natural resources and reducing the associated environmental impact.
Waste Reduction: Recycling lithium-ion batteries prevents them from ending up in landfills or incineration, minimizing the release of hazardous materials into the environment.
Energy Savings: Extracting metals from recycled battery components requires less energy than producing metals from raw materials. This leads to reduced greenhouse gas emissions and a more sustainable energy footprint.
Circular Economy: Metal extraction from lithium-ion batteries promotes a circular economy by reintroducing valuable metals into the production cycle, reducing the dependency on virgin resources and supporting a more sustainable supply chain.
Conclusion
The metal extraction from lithium-ion batteries is a crucial step in battery recycling. By recovering valuable metals like lithium, cobalt, nickel, and others, we can reduce resource depletion, minimize environmental impact, and foster a circular economy. As the demand for lithium-ion batteries continues to rise, it is essential to invest in efficient and environmentally friendly recycling technologies. By doing so, we can ensure the responsible management of battery waste and contribute to a more sustainable future.