Lithium-ion batteries are manufactured in sets of electrodes and then assembled in cells. Active material is mixed with polymer binders, conductive additives, and solvents to form a slurry that is then coated on a current collector foil and dried to remove the solvent and create a porous electrode coating.
There is no single lithium ion battery. With the variety of materials and electrochemical couples available, it is possible to design battery cells specific to their applications in terms of voltage, state of charge use, lifetime needs, and safety. Selection of specific electrochemical couples also facilitates the design of power and energy ratios and available energy.
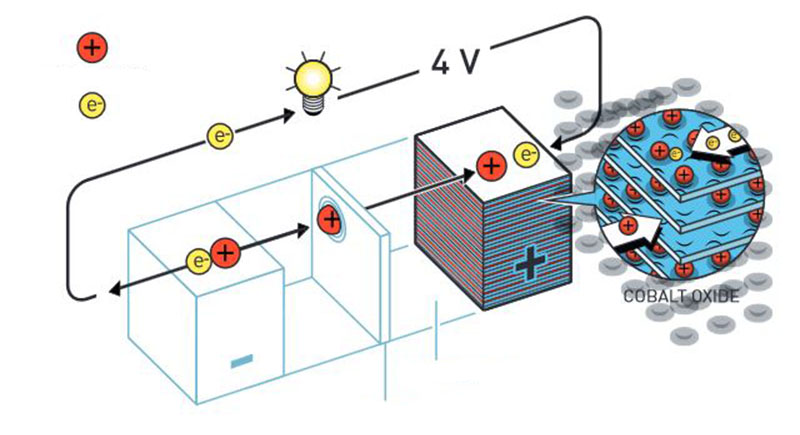
The lithium ion battery was made possible by the discovery of lithium cobalt oxide (LiCoO2), which allows the extraction of lithium ions and creation of large amounts of vacancies (without a crystal change) up to the removal of half of the existing ions. The pairing of LiCoO2 with graphite allows the intercalation of lithium ions between the graphene layers that occupy the interstitial site between every hexagonal ring of carbon atoms (Besenhard and Schöllhorn 1976; Mizushima et al. 1980; Whittingham 1976).
The search for new cathode materials is driven in part by important disadvantages of LiCoO2. The battery has a core temperature of 40–70°C and may be susceptible to some low-temperature reactions. But at 105–135°C it is very reactive and an excellent oxygen source for a safety hazard called a thermal runaway reaction, in which highly exothermic reactions create temperature spikes and accelerate rapidly with the release of extra heat (Roth 2000)
In general, a battery cell is made up of an anode, cathode, separator and electrolyte which are packaged into an aluminium case. The positive anode tends to be made up of graphite which is then coated in copper foil giving the distinctive reddish-brown colour. The negative cathode has sometimes used aluminium in the past, but nowadays is a mix of cobalt, nickel and manganese leading to a mixed-metal oxide material. The electrolyte is a lithium salt in a dissolved solution known as a solvent (which is usually ethylene carbonate). Finally, the separator (which – as its name suggests – physically separates the anode and cathode) is a micro-porous compound which is usually plastic-based such as polypropylene or polyethylene.
Lithium-ion battery packs come in all shapes and sizes, but they all look about the same on the inside. If you were to take apart a laptop battery pack you would find the following:
- The lithium-ion cells can be either cylindrical batteries that look almost identical to AA cells, or they can be prismatic, which means they are square or rectangular The computer, which comprises:
- One or more temperature sensors to monitor the battery temperature
- A voltage converter and regulator circuit to maintain safe levels of voltage and current
- A shielded notebook connector that lets power and information flow in and out of the battery pack
- A voltage tap, which monitors the energy capacity of individual cells in the battery pack
- A battery charge state monitor, which is a small computer that handles the whole charging process to make sure the batteries charge as quickly and fully as possible.